How do I resuspend my oligos?
If you receive your oligos dry, you will need to resuspend them in aqueous solution before use. Most oligos are relatively easy to resuspend; however, those modified with fluorophores or hydrophobic molecules may require more time to become completely solubilized.
Below are some useful recommendations for resuspending oligos from our scientists. For guidance on oligonucleotide storage or additional helpful tips about handling newly received oligos, see the articles, Storing oligos: 7 things you should know and My oligos have arrived: Now what?
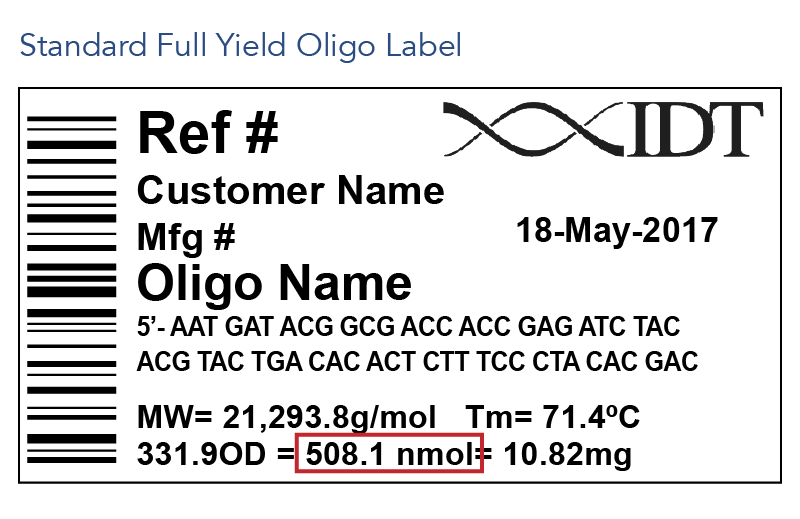
- Our standard recommendation is to resuspend oligos to a 100 µM stock concentration, as this is a simple concentration to make and is highly versatile for subsequent dilution purposes. However, the resuspension concentration is up to your discretion and depends on how the oligo will be used in future experiments. Oligo yield information is conveniently located on the oligo tube label (Figure 1) and specification sheet (Figure 2), which can be used to calculate how much buffer to use for resuspension at your desired concentration. See ‘Calculating buffer volume when resuspending oligos’ below for additional details.
- During the dry-down process, oligos may form a thin clear film or a white flakey pellet at the bottom of the tube. Since the pellet can become dislodged during shipping, we recommend that you briefly centrifuge every oligo before opening the tube. Skipping this step could result in yield loss because oligo pellets that are not at the bottom of the tube could accidentally fly out of the tube when the cap is opened.
- If resuspension is difficult, try heating the oligo at 55°C for 1–5 minutes, then vortex thoroughly. If any precipitates remain, they are likely either trityl groups (flakey appearance) or the controlled-pore glass (CPG; a pellet at bottom of tube) on which the oligo was synthesized. These should not impact the oligos but can be removed easily—just run the resuspended oligo through a column (e.g., Sephadex® G-50 column [Cytiva]) or transfer the supernatant (which contains the oligo) to a new tube.
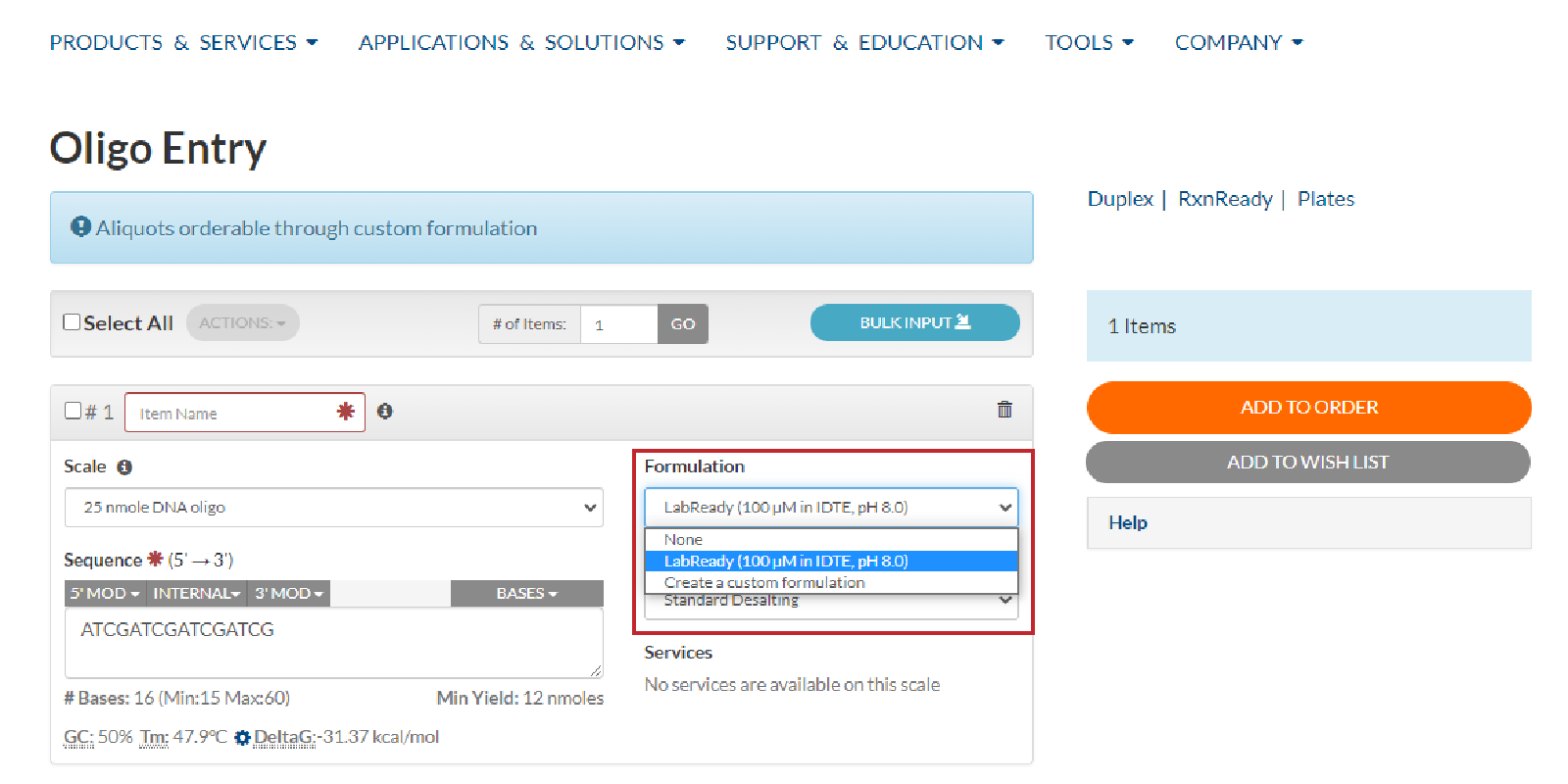
- IDT oligonucleotides (both DNA and RNA) are typically shipped dry. However, you can request to have your DNA oligos resuspended prior to shipment. To obtain resuspended oligos, use the Normalization dropdown menu (Figure 3) on the Oligo Entry ordering tool. Here, you can select LabReady (100 µM in IDTE, pH 8.0), or create a custom formulation to your specifications.
- Do not expose your oligos to highly acidic or basic conditions at any time during resuspension. We recommend resuspending oligos in a TE buffer solution, such as IDTE, to maintain a constant pH that supports oligo stability (IDTE is available from IDT at pH 7.5 and pH 8.0). Alternatively, resuspend oligos in nuclease-free, sterile water, pH 7.0 (HPLC- or molecular biology-grade is preferable, such as IDT's Nuclease-Free Water). We recommend avoiding DEPC-treated water or water from deionizing systems when resuspending oligos as they are often acidic, which can cause depurination and damage DNA oligos over time.
Calculating buffer volume when resuspending oligos
To make a 100 µM storage solution:
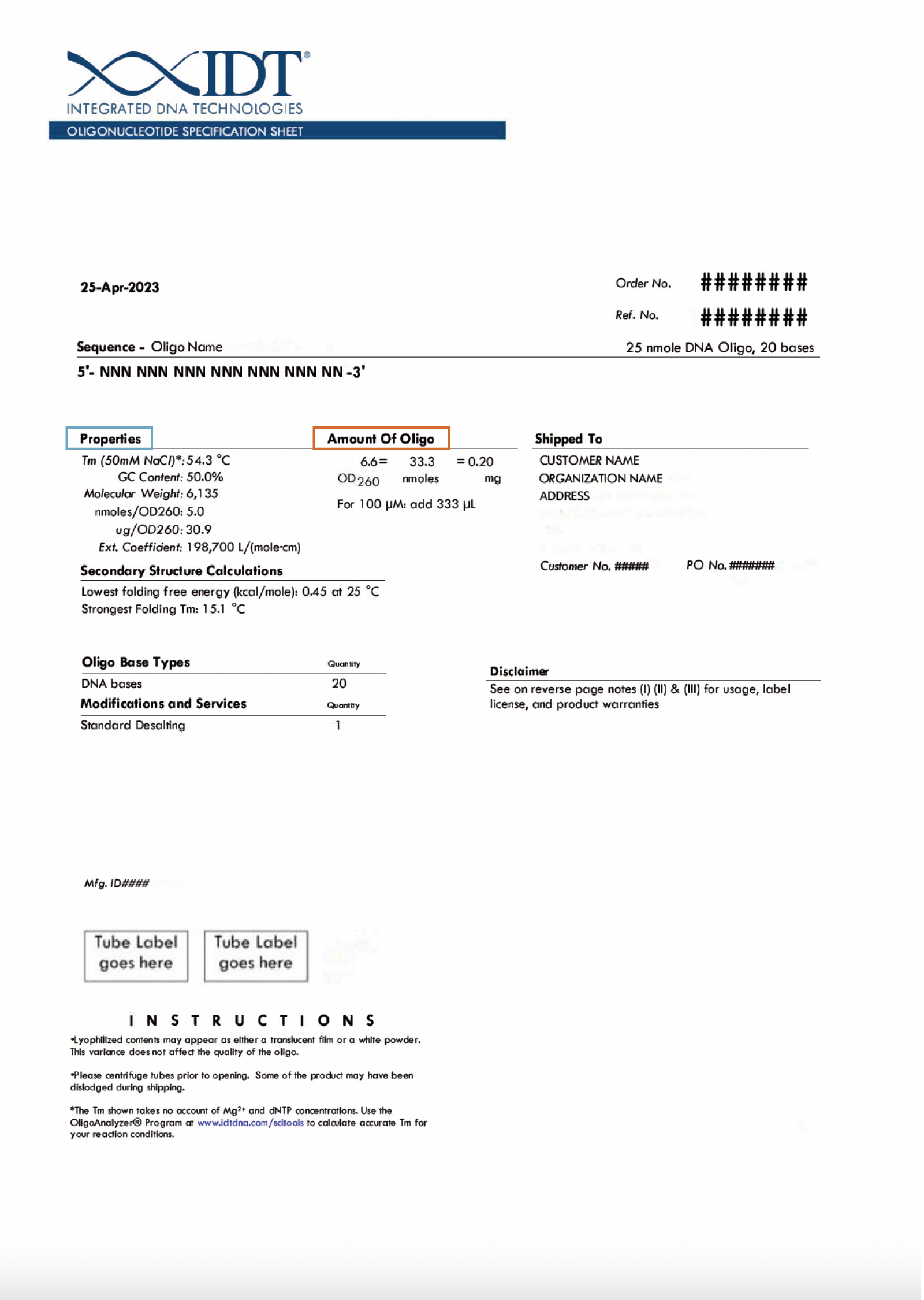
- Find the oligo yield information in nmol on your tube label (Figure 1) or in the "Amount of oligo" section of your specification sheet (Figure 2).
- Multiply this number by 10.
- The resulting product is the amount of buffer needed, in µL, to prepare a 100 µM solution. For example, if yield is 9 nmol, 90 µL of buffer is needed to make a 100 µM solution.
Note: At 100 µM concentration, 1 µL solution contains 100 pmol of oligo. PCRs typically require 10–50 pmol of each primer per reaction.
Alternative storage and working solution concentrations can be made easily using our Resuspension Calculator and Dilution Calculator, as described below.
To make storage solutions at other concentrations:
- Find the oligo yield information on the tube label (Figure 1) or specification sheet (Figure 2). This will be listed in 3 forms—optical density (OD), nanomoles (nmol), and mass (mg).
- Enter one of these yield measurements in the Quantity box of the Resuspension Calculator and select the appropriate units.
- In the Final Concentration box, enter your desired stock concentration and again select the appropriate units.
- Click Calculate. The tool will determine the volume of buffer/water needed to create the desired stock.
Note: Based on the units selected, you may be required to enter the molar extinction coefficient and/or the molecular weight of your oligo. This information can be found in the "Properties" section of your oligo specification sheet (Figure 2).
To make a working stock from storage stock:
Working stocks can be made easily from storage stocks using the IDT oligo Dilution Calculator.
- Enter the starting concentration and volume, then select the appropriate units.
- Enter the final (desired) concentration and volume, and select the appropriate units.
- Click Calculate. The tool will determine the volume of buffer/water needed to dilute your storage stock to the desired working stock concentration.
Note: Based on the units selected, you may be required to enter the molecular weight of your oligo. This information can be found in the "Properties" section of your oligo specification sheet (Figure 2).
For further assistance in resuspending and diluting your oligos, please contact us.
Additional calculation tips and formulas
As mentioned above, IDT provides oligo yield information in 3 forms—optical density (OD), nanomoles (nmol), and mass (mg). Researchers may also be interested in using copy number to describe oligo amounts. Converting between these different units of measure can be useful for various laboratory applications. Although it is quick and convenient to use our online calculators, it can be helpful to understand the math behind the calculations. Here are some example equations that illustrate how we can calculate oligo concentration, determine copy number, and easily convert from one unit of measure to another.
Calculating micrograms
If you know how many nanomoles of product you have and the molecular weight (g/mol) of your oligo, you can use this information to calculate the amount of oligo in micrograms. Multiply the oligo amount in nmol by the molecular weight of your oligo, then divide the result by 1000 (Figure 4).
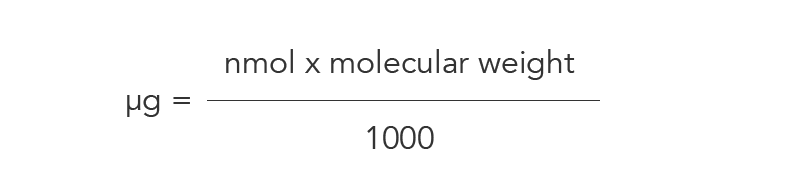
Calculating copy number
Avogadro’s number (6.022 x 1023) is the number of molecules in one mole of a substance. If you know how many moles of oligo you have, you can use Avogadro’s number to calculate how many individual oligo molecules (copy number) are in your sample or stock solution (Figure 5). IDT typically delivers oligos in nmol or µmol amounts, so you may need to adjust your calculations accordingly (1 nmol = 1 x 10-9 mol; 1 µmol = 1 x 10-6 mol).

Calculating concentration
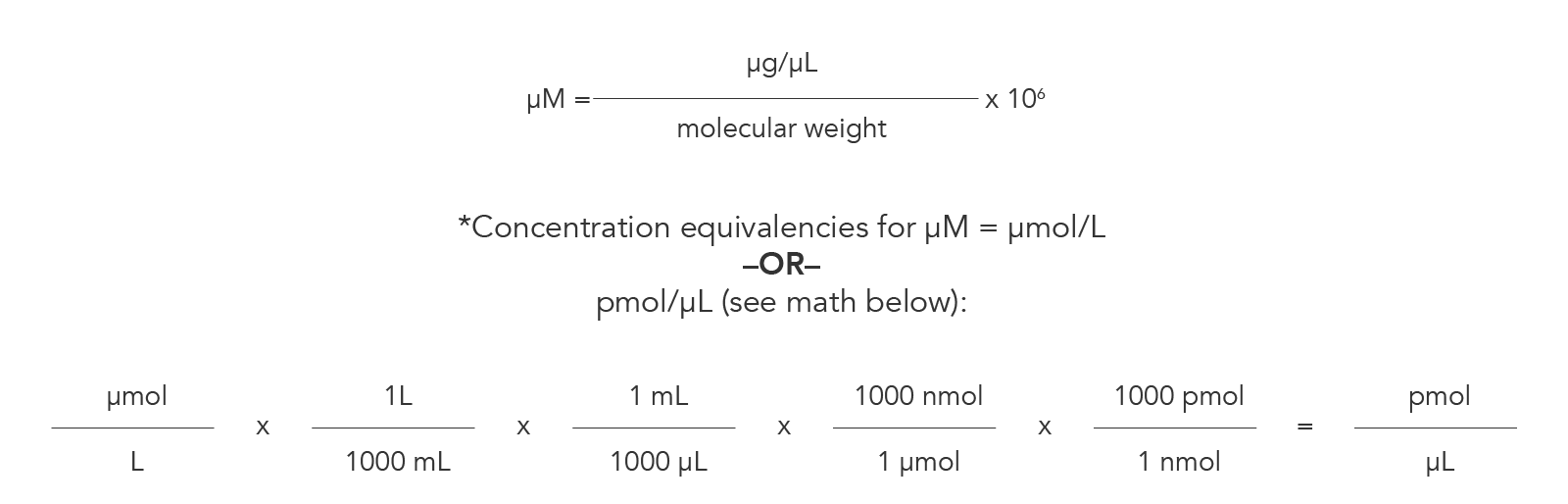
Oligo concentration describes the amount of oligos present in a specified volume, such as one liter or microliter. This is commonly conveyed as micromolarity (µM), or the number of micromoles (1 x 10-6 moles) per liter. The first equation shown in Figure 6 demonstrates the mathematical relationship between molarity, mass, volume, and molecular weight. Dimensional analysis is a useful strategy for converting between different concentration equivalencies, as illustrated in the second equation in Figure 6.
RUO—For research use only. Not for use in diagnostic procedures. Unless otherwise agreed to in writing, IDT does not intend for these products to be used in clinical applications and does not warrant their fitness or suitability for any clinical diagnostic use. Purchaser is solely responsible for all decisions regarding the use of these products and any associated regulatory or legal obligations. Doc ID: RUO23-1765_001


 Processing
Processing