Research into applications using CRISPR-Cas systems for genome editing is expanding quickly. This presents a need for effective reagents and protocols for a wide variety of model systems beyond mammalian cultured cells.
Advantages of Alt-R CRISPR guide RNAs and proteins for genome editing
CRISPR guide RNAs. Use Alt-R CRISPR guide RNAs (gRNAs) to direct potent, on-target genome editing. These length-optimized RNAs are chemically synthesized, which allows addition of modifications for increased nuclease resistance and reduced innate immune responses.
CRISPR proteins. Choose from several recombinant variants of Cas9 (Streptococcus pyogenes), as well as Cas12a (formerly Cpf1) (Acidaminococcus sp. BV3L6) endonucleases. Alt-R S.p. Cas9 Nuclease V3 is suitable for most genome editing studies. Some experiments may benefit from use of Alt-R S.p. HiFi Cas9 Nuclease, a high-fidelity nuclease that has been engineered to reduce off-target effects while retaining the on-target potency of wild-type Cas9. Alt-R Cas12a (Cpf1) nucleases provide additional genome editing target sites, because they recognize an AT-rich protospacer adjacent motif (PAM) rather than the CG-rich Cas9 PAM sequence. If you are interested in knock-in experiments or other applications requiring homology-directed repair (HDR), consider using an Alt-R Cas9 nickase to create single-strand breaks. Use of a nickase variant with a pair of guide RNAs reduces off-target effects by requiring specific binding of 2 ribonucleoproteins (RNPs). However, you will need 2 potent CRISPR target sites at an appropriate distance from your HDR target site.
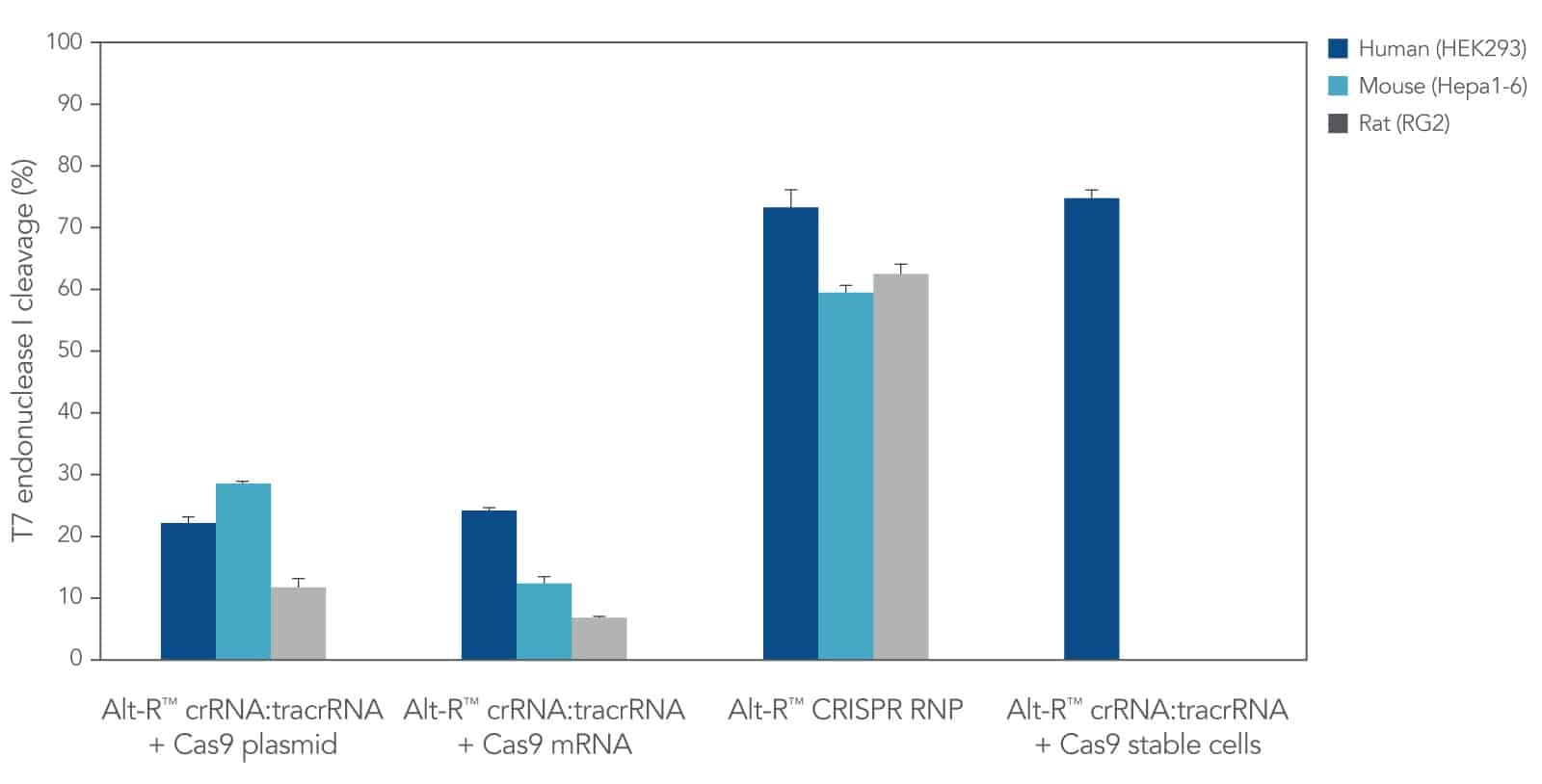
CRISPR RNPs. While Alt-R CRISPR guide RNAs are compatible with CRISPR proteins from any source (e.g., cells that stably express CRISPR protein, or CRISPR proteins expressed from DNA or mRNA constructs), we recommend delivery of CRISPR reagents as RNPs by lipofection (e.g., Figure 1), electroporation, or microinjection. Cas9 RNPs are compatible with all 3 methods; however, for Cas12a RNPs, we recommend electroporation and microinjection delivery methods.
Advantages of using RNPs
- Efficient, on-target genome editing
- Precise control of editing complex delivery—cells will not contain templates that express unregulated levels of CRISPR RNAs or endonucleases
- Lower activation of cellular immune responses and, therefore, reduced toxicity—toxicity is often observed when using in vitro transcribed Cas9 mRNA or single guide RNAs (sgRNA)
Protocols and starting points are increasingly available
IDT scientists have developed detailed lipofection and electroporation protocols for using the Alt-R CRISPR-Cas9 System and the Alt-R CRISPR-Cas12a System in mammalian cells (Table 1). With help from our collaborators, we also make user-submitted methods available for genome editing in other model systems. These protocols and methods are a good starting point for protocol optimization for your studies, and often offer tips for all aspects of genome editing experiments, from growth conditions through genome editing detection.
Table 1. Alt-R CRISPR System protocols and user methods.*
| Model system | Transfection method | IDT protocols |
| Cultured cells | Lipofection | [1] |
| Cultured cells | Electroporation | [2–5] |
| In vitro | — | [6] |
| Model system | Transfection method | User-submitted methods |
| Human pluripotent or embryonic stem cells | Electroporation | [7] |
| Mouse zygote | Electroporation | [8] |
| Mouse zygote | Microinjection | [9] |
| Zebrafish embryo | Microinjection | [10] |
| C. elegans | Injection | [11] |
* Visit www.idtdna.com/protocols to access the most up-to-date list of protocols and user-submitted methods.
Do you have a protocol to share?
We are always interested in learning about successful editing in different model systems and would like to serve as your resource for information about genome editing applications. If you have a novel protocol for using Alt-R CRISPR RNA and/or nucleases that you would like to share with the research community, email us at CRISPR@idtdna.com to start the discussion.
References
- Integrated DNA Technologies. Alt-R CRISPR-Cas9 System: Cationic lipid delivery of CRISPR ribonucleoprotein complex into mammalian cells. 2017; Coralville, IA. Integrated DNA Technologies.
- Integrated DNA Technologies. Alt-R CRISPR-Cas9 System: Delivery of ribonucleoprotein complexes in HEK-293 cells using the Neon® Transfection System. 2017; Coralville, IA. Integrated DNA Technologies.
- Integrated DNA Technologies. Alt-R CRISPR-Cas9 System: Delivery of ribonucleoprotein complexes into Jurkat T cells using the Bio-Rad Gene Pulser® Xcell™ Electroporation System. 2017; Coralville, IA. Integrated DNA Technologies.
- Integrated DNA Technologies. Alt-R CRISPR-Cpf1 System: Delivery of ribonucleoprotein complexes in HEK-293 cells using the Amaxa Nucleofector System. 2017; Coralville, IA. Integrated DNA Technologies.
- Integrated DNA Technologies. Alt-R CRISPR-Cpf1 System: Delivery of ribonucleoprotein complexes in Jurkat T cells using the Neon Transfection System. 2017; Coralville, IA. Integrated DNA Technologies.
- Integrated DNA Technologies. Alt-R CRISPR-Cas9 System: In vitro cleavage of target DNA with ribonucleoprotein complex. 2017; Coralville, IA. Integrated DNA Technologies.
- Moore K, Martin J. Electroporation of human pluripotent or embryonic stem cells with CRISPR reagents. 2017; Coralville, IA. Integrated DNA Technologies.
- Hashimoto M, Takemoto T. Mouse zygote electroporation: Ribonucleoprotein delivery using the Alt-R CRISPR-Cas9 System. 2017; Coralville, IA. Integrated DNA Technologies.
- Quadros R, Harms D, Gurumurthy CB. Mouse zygote microinjection: Ribonucleoprotein delivery using the Alt-R CRISPR-Cas9 System. 2016; Coralville, IA. Integrated DNA Technologies.
- Essner J. Zebrafish embryo microinjection: Ribonucleoprotein delivery using the Alt-R CRISPR-Cas9 System. 2016; Coralville, IA. Integrated DNA Technologies.
- Kohler S, Dernburg A. C. elegans injection: Ribonucleoprotein delivery using the Alt-R CRISPR-Cas9 System. 2016; Coralville, IA. Integrated DNA Technologies.
For research use only. Not for use in diagnostic procedures. Unless otherwise agreed to in writing, IDT does not intend these products to be used in clinical applications and does not warrant their fitness or suitability for any clinical diagnostic use. Purchaser is solely responsible for all decisions regarding the use of these products and any associated regulatory or legal obligations. Doc ID: RUO22-1429_001